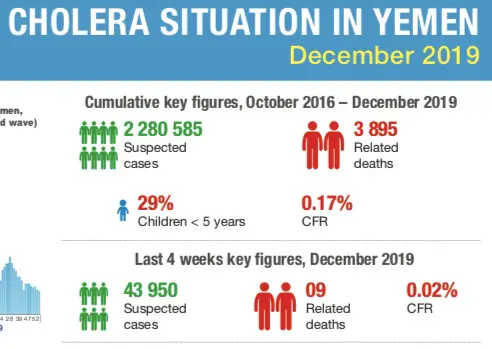
While the planet is in the midst of the COVID-19 pandemic, and yes it is serious, with 67 194 reported cases thus far and 1 527 deaths in 27 countries around the world (if you include Hong Kong and Macau with China), spare a thought for the rather serious cholera epidemic in civil war ravaged Yemen. A country ill prepared for a disease epidemic of this magnitude. Statistics released by the World Health Organization in December 2019 (Figure 1) show a total of 2 280 585 reported cases of cholera in Yemen since this epidemic began in October 2016, with 3 895 deaths. Into the new decade with another 6 856 cases reported between 1-7 January 2020, and one death – the situation is serious in Yemen too, a country in dire circumstances politically, with poor infrastructure and a healthcare system that cannot handle an epidemic of such epic proportion. However, the struggles of Yemen against an ancient disease that has been the scourge of humanity for millennia, is off the radar to most people – let us not forget about them.
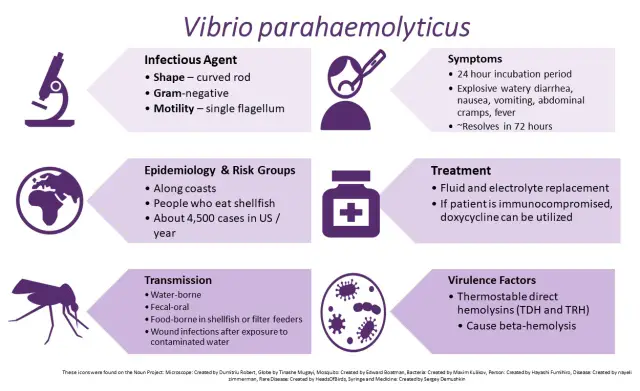
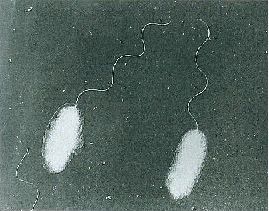
Recently (26 January 2020), I posted an overview of pathogenic vibrios. While the waterborne Vibrio cholerae has been, is and almost certainly will continue to plauge human society, let’s turn our attention to Vibrio parahaemolyticus (Figure 2). Unlike V. cholerae, V. parahaemolyticus has been known for a mere 70 years. Although not to the grand scale of V. cholerae, V. parahaemolyticus has firmly established itself as a key foodborne pathogen. Let me explain, using text that I have mostly lifted from a report I wrote in 1998, but reviewed, revised and updated where appropriate.
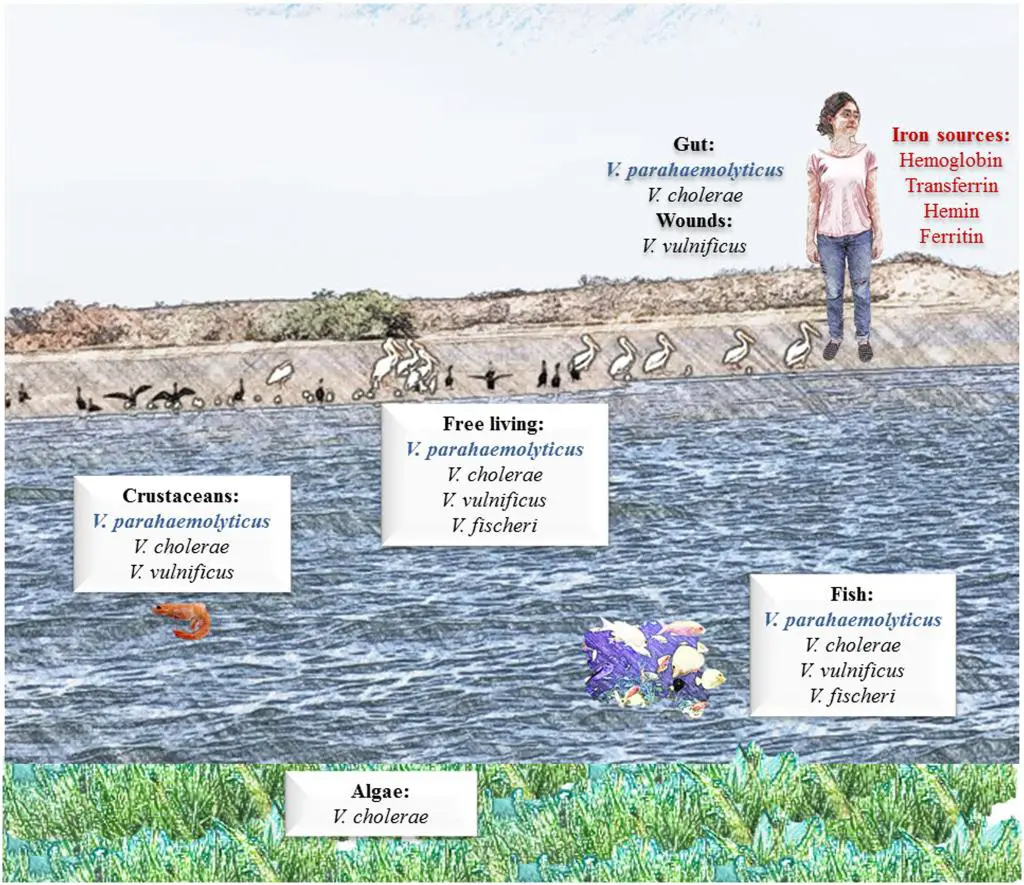
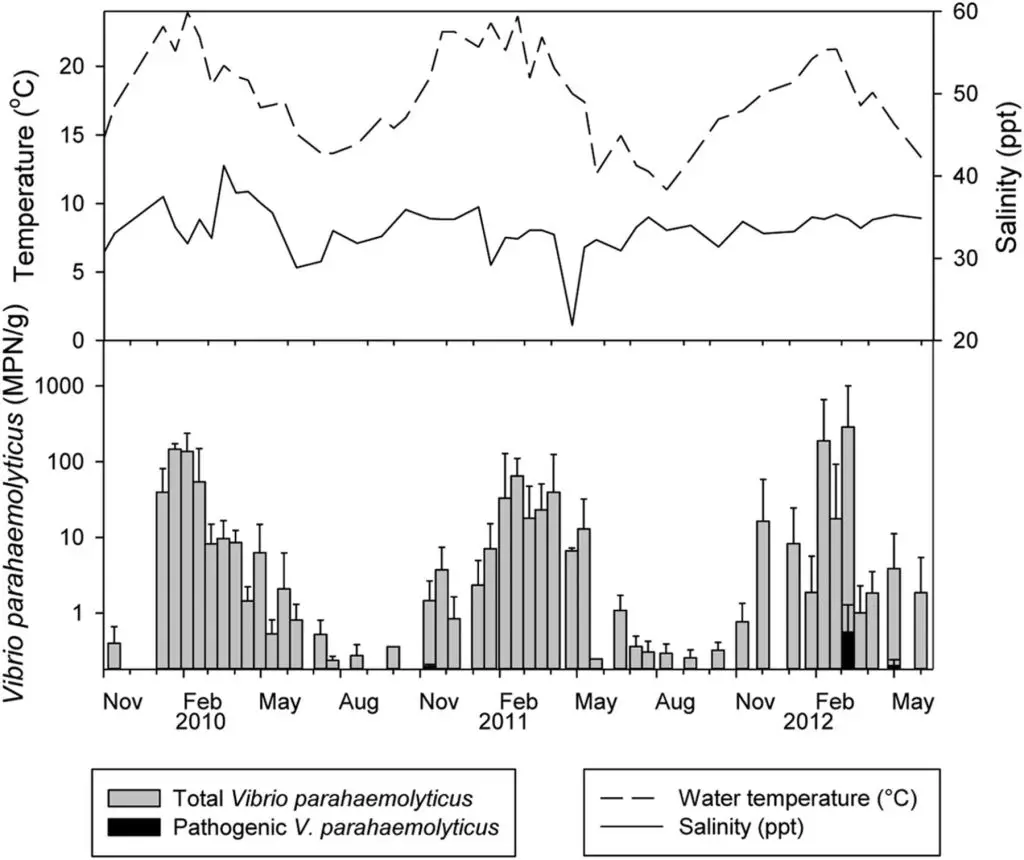
V. parahaemolyticus is a common marine inhabitant (Figure 3) worldwide that has been recognised as an agent of gastroenteritis for the past 70 years. The first documented case of foodborne disease by V. parahaemolyticus occurred in Osaka, Japan – the year was 1950 (Joseph et al., 1982). Foodborne acquisition of this organism is most significant from marine environments and it is recognised as the most important seafood-borne pathogen, with the highest incidence of implication in foodborne disease cases involving seafood. In fact, in some countries, such as China, V. parahaemolyticus is the leading cause of foodborne disease (Wu et al., 2018). In addition to its prevalence in marine environments, it has been shown to be present in freshwater (Myatt and Davis, 1989) and in seafood caught from such areas (Sarkar et al., 1985), though this appears rare. Although widespread, numbers vary seasonally (Figure 4), especially in temperate regions, where the bacteria are present in greater numbers during the summer months and this has been closely correlated with reported cases (Kelly and Stroh, 1988; Pan et al., 1997). However, shellfish, particularly oysters have been a common source of the infection (CDC, 1988; CDC, 1991; Klontz et al., 1993) and this is true not only for V. parahaemolyticus, but for many other Vibrios species (Hlady and Klontz, 1996).
Gastroenteritis is the condition most often experienced when people are infected with V. parahaemolyticus, with diarrhoea, abdominal cramps and nausea seen in at least 70% of cases (ICMSF, 1995). Other disease syndromes have been reported, such as a cholera-like disease (Mhalu et al., 1982), septicaemia (Rabinowitch et al., 1993), wound infections (Plotkin et al., 1994) and dysentery (Gilman et al., 1980). It is often self-limiting and rarely fatal. However, deaths have been reported (Thamlikitkul, 1990; Kraa, 1995).
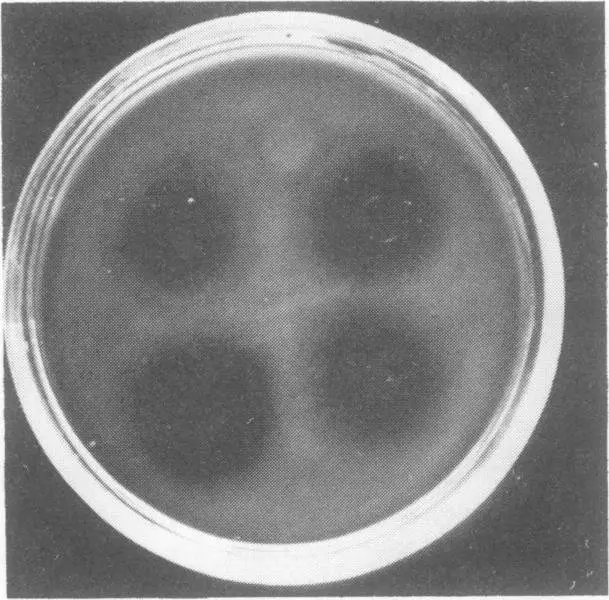
A key aspect of both isolation of V. parahaemoyticus and relationship to human pathogenicity is the so-called “Kanagawa phenomenon”. The Kanagawa phenomenon is a beta-haemolytic reaction (Figure 5) caused by the thermostable direct haemolysis (Okuda & Nishibuchi, 1998) that takes place on a specific medium, and is closely associated with pathogenicity. Growth conditions for production of the heat-stable haemolytic are influenced by a range of growth parameters, including pH and type of carbohydrate in the medium (Chun et al. 1975).
The testing of clinical samples for Vibrio spp. by laboratories in coastal areas, especially those in warmer regions, should be a routine procedures when a patient presents with symptoms which are typical of Vibrio infection, such as gastroenteritis. This is due to the abundance of V. parahaemolyticus in the marine environment and the potential that all seafood could be contaminated. Despite this abundance, 99.5% of environmental isolates of V. parahaemolyticus are reported to be Kanagawa negative (Miyamoto et al. (1969), meaning the vast majority of environmental isolates are non-pathogenic too humans. This is significant because 88% to 95% of V. parahaemolyticus isolates from patients infected with this species are Kanagawa positive (Miyamoto et al., 1969; Wong et al., 2000), with reports on infection by Kanagawa negative V. parahaemolyticus rare (Johnson et al., 1984). Despite this, Kanagawa positive isolates are considered virulent isolates (Okuda & Nishibuchi, 1998).
In seven decades, Vibrio parahaemolyticus has established as a pathogen of global significance. Its ubiquity in the marine environment ensures that it remains a leading cause of foodborne illness, in various parts of the world.
References
Centers for Disease Control (CDC. (1991). Gastroenteritis associated with consumption of raw shellfish–Hawaii, 1991. MMWR. Morbidity and mortality weekly report, 40(18), 303.
Centers for Disease Control and Prevention (CDC). (1998). Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters–Pacific Northwest, 1997. MMWR. Morbidity and mortality weekly report, 47(22), 457.
Chun, D. O. K. I., Chung, J. K., Tak, R. Y. U. N. B. I. N., & Seol, S. Y. (1975). Nature of the Kanagawa phenomenon of Vibrio parahaemolyticus. Infection and immunity, 12(1), 81-87.
Cruz, C. D., Hedderley, D., & Fletcher, G. C. (2015). Long-term study of Vibrio parahaemolyticus prevalence and distribution in New Zealand shellfish. Applied and Environmental Microbiology, 81(7), 2320-2327.
Gilman, R. H., Spira, W. M., Rabbani, G. H., & Al-Mahomod, A. (1980). Invasive E. coli and V. parahaemolyticus a rare cause of dysentery in Dacca. Transactions of The Royal Society of Tropical Medicine and Hygiene, 74(5), 688-689.
Hlady, W. G., & Klontz, K. C. (1996). The epidemiology of Vibrio infections in Florida, 1981–1993. Journal of Infectious Diseases, 173(5), 1176-1183.
ICMSF/International Commission on Microbiological Specifications for Foods. (1995). Roberts, T., Baird-Parker, A. & Tompkin, R. (Eds.). Microorganisms in Foods 5. London, England: Blackie.
Johnson, D. E., Weinberg, L., Ciarkowski, J., West, P., & Colwell, R. R. (1984). Wound infection caused by Kanagawa-negative Vibrio parahaemolyticus. Journal of Clinical Microbiology, 20(4), 811-812.
Joseph, S. W., Colwell, R. R., & Kaper, J. B. (1982). Vibrio parahaemolyticus and related halophilic vibrios. CRC Critical Reviews in Microbiology, 10(1), 77-124.
Kelly, M. T., & Stroh, E. M. (1988). Temporal relationship of Vibrio parahaemolyticus in patients and the environment. Journal of Clinical Microbiology, 26(9), 1754-1756.
KLONTZ, K. C., WILLIAMS, L., BALDY, L. M., & CAMPOS, M. (1993). Raw oyster-associated Vibrio infections: linking epidemiologic data with laboratory testing of oysters obtained from a retail outlet. Journal of food protection, 56(11), 977-979.
Kraa, E. (1995). Surveillance and epidemiology of foodborne illness in NSW, Australia. Food Australia, 47(9), 418.
Mhalu, F. S., Yusufali, A. M., Mbwana, J., & Nyambo, R. (1982). Cholera-like diseases due to Vibrio parahaemolyticus. The Journal of tropical medicine and hygiene, 85(4), 169-171.
Miyamoto, Y., Kato, T., Obara, Y., Akiyama, S., Takizawa, K., & Yamai, S. (1969). In vitro hemolytic characteristic of Vibrio parahaemolyticus: its close correlation with human pathogenicity. Journal of bacteriology, 100(2), 1147.
Myatt, D. C., & Davis, G. H. (1989). Isolation of medically significant Vibrio species from riverine sources in south east Queensland. Microbios, 60(243), 111-123.
Okuda, J., & Nishibuchi, M. (1998). Manifestation of the Kanagawa phenomenon, the virulence‐associated phenotype, of Vibrio parahaemolyticus depends on a particular single base change in the promoter of the thermostable direct haemolysin gene. Molecular microbiology, 30(3), 499-511.
Pan, T. M., Wang, T. K., Lee, C. L., Chien, S. W., & Horng, C. B. (1997). Food-borne disease outbreaks due to bacteria in Taiwan, 1986 to 1995. Journal of clinical microbiology, 35(5), 1260-1262.
Plotkin, B. J., Kilgore, S. G., & McFarland, L. (1990). Polyvibrio infections: Vibrio vulnificus and Vibrio parahaemolyticus dual wound and multiple site infections. Journal of Infectious Diseases, 161(2), 364-365.
Rabinowitch, B. L., Nam, M. H., Levy, C. S., & Smith, M. A. (1993). Vibrio parahaemolyticus septicemia associated with water-skiing. Clinical infectious diseases, 16(2), 339-340.
Sarkar, B. L., Nair, G. B., Banerjee, A. K., & Pal, S. C. (1985). Seasonal distribution of Vibrio parahaemolyticus in freshwater environs and in association with freshwater fishes in Calcutta. Appl. Environ. Microbiol., 49(1), 132-136.
Thamlikitkul, V. I. S. A. N. U. (1990). Vibrio bacteremia in Siriraj Hospital. Journal of the Medical Association of Thailand= Chotmaihet thangphaet, 73(3), 136-139.
Wong, H. C., Liu, S. H., Ku, L. W., Lee, I. Y., Wang, T. K., Lee, Y. S., … & Shih, D. Y. C. (2000). Characterization of Vibrio parahaemolyticus isolates obtained from foodborne illness outbreaks during 1992 through 1995 in Taiwan. Journal of food protection, 63(7), 900-906.
Wu, Y. N., Liu, X. M., Chen, Q., Liu, H., Dai, Y., Zhou, Y. J., … & Chen, Y. (2018). Surveillance for foodborne disease outbreaks in China, 2003 to 2008. Food Control, 84, 382-388.