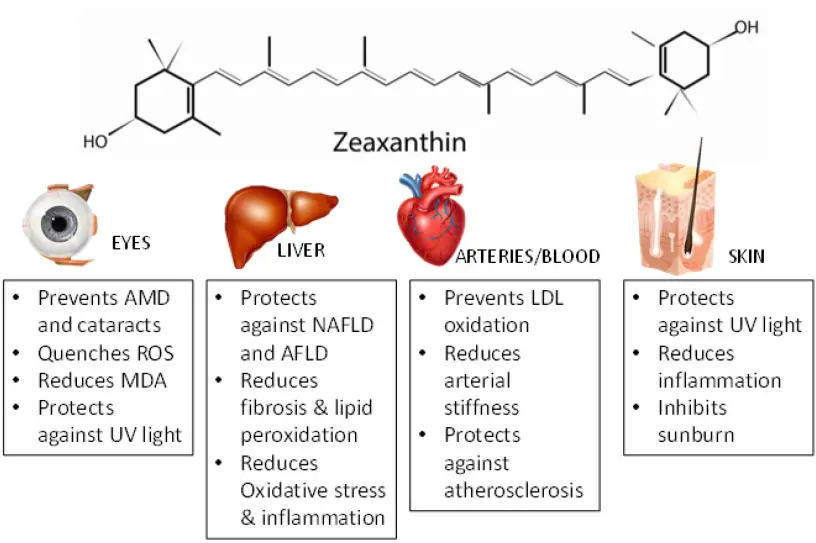
This blog article is a small extract from an Honours research proposal (that didn’t proceed), written in 2008 by one of my students at RMIT University, Weny Tjong. I have provided some current updates in particular areas. Even though this article would be considered within the realm of industrial microbiology and biotechnology, the connection to food microbiology and food science in general is because this purified zeaxanthin was to be used to fortify food products.
One potential Sphingobacterium sp. to be considered as a source of zeaxanthin is Sphingobacterium multivorum (Figure 1) as it is a non fastidious and (generally) non-pathogenic bacterium that rapidly accumulates zeaxanthin through their fermentation (Sajilata, Singhal & Kamat, 2008). Although generally pathogenic, there are a handful of reports of disease caused by the species (for example: Abro et al., 2016 and Mendes et al., 2016). Bacteria are seen to have great potential for industrial production of carotenoids (Ram et al., 2020). The suggestion of its rapid zeaxanthin accumulation is very much supported by Alcantara and Sanchez (1999), and Garnett et al. (1998). The ATCC number of this bacterial strain is 55238. It is reported that under proper fermentation conditions, zeaxanthin can be synthesized by this bacterium with virtually no significant amount of other carotenoids (Gierhart, 1994). Hence, the difficulty in purifying zeaxanthin from its closely related carotenoids can be eliminated with the use of Sphingobacterium multivorum and its descendants.
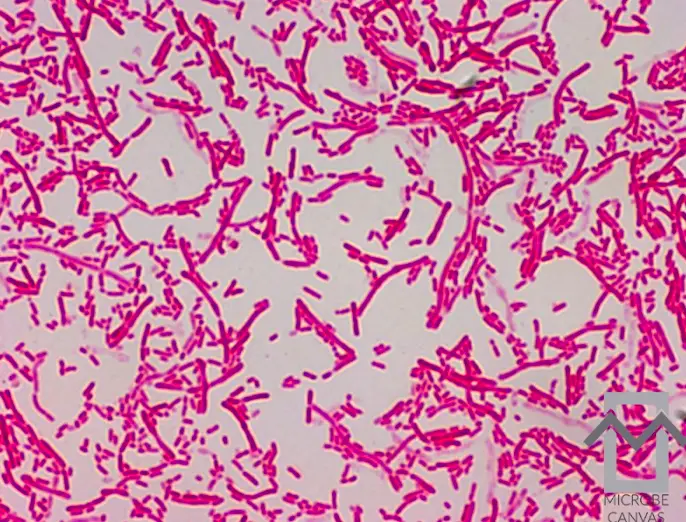
Sphingobacterium multivorum characteristics
With a few exceptions, Sphingobacterium sp. can be categorized into two categories: strongly proteolytic – digest gelatine, casein and coagulated serum- and non proteolytic (Gierhart, 1994). S. meningosepticum (biovar IIa) and S. indologenes (viovar IIb) are always proteolytic; other Flavobacteria are not. Sphingobacterium multivivorum is non proteolytic.
S. multivorum grows in smooth, non pigmented colonies of size less than 1 mm on a 1 day sheep blood agar plate (Gierhart, 1994) (Figure 2). They are oxidase-positive, catalase-positive, Gram negative rods, non-motile, strongly sucrose-positive, always mannitol-negative and ethanol-negative, and urea-positive. The plate shows no zones of inhibition surrounding penicillin, vancomycin, and polymyxin disks.
S. multivorum zeaxanthin production
The stereoisomeric isomers of zeaxanthin appear to be small, subtle and almost insignificant. Nonetheless, when it comes to retinal tissue, only the 3R-3’R zeaxanthin stereoisomer (Figure 3) is properly taken up and used by human retinal cells (Garnett et al., 1998).

Traces quantities of the meso-zeaxanthin isomers have been reportedly to be found in retinal tissues (Garnett et al., 1998). However, it is argued that these trace quantities are probably the result of molecular conversion of the lutein precursors under some conditions.
S. multivorum only produces desired R-R isomers and do not synthesize other undesired S–S or S-R isomers of zeaxanthin and significant quantities of other carotenoids such as β-carotene or lutein, which might compete against zeaxanthin for the alimentary uptake after oral ingestion (Garnett et al. 1998). The concentration of the zeaxanthin obtained after the cell fermentation can be concentrated to approximately 5 to 20% by simple and inexpensive steps such as solvent extraction, and/or purified to much higher levels by other methods if desired.
Factors That Affect Zeaxanthin Production in Sphingobacterium sp.
- Nitrogen and carbon sources
Studies were done by Alcantara and Sanchez in 1999 whereby the growth of Sphingobacterium sp. utilizing different carbon and nitrogen sources were observed. The result of this observation is shown in Figure 6. Sucrose and both asparagine and glutamine were found to be the best stimulator for growth and pigment formation. The carotenoid production and glucose consumption increased in respect to asparagine concentration that depletes as it is used by Sphingobacterium sp. as their primary source of nitrogen for growth and zeaxanthin production. The presence of asparagine together with high glucose concentrations in fact decreases pigment production although the biomass formation is unaffected (Alcantara and Sanchez, 1999). However, without glucose, asparagine could not support growth of the cells and subsequent zeaxanthin production.
(Alcantara & Sanchez, 1999)
CD: Chemically defined
OD: Optical density
^ Effect of different carbon and nitrogen sources on growth and zeaxanthin production
- The Presence of Corn Steep Liquor
Corn steep liquor is known to be beneficial on for the growth and pigment synthesis of Sphingobacterium sp. (Gierhart, 1994). It is thought that this corn steep liquor contains good amounts of amino acids and minerals necessary for the growth of these microorganisms. Without any special measurement taken, the fermentation of Sphingobacterium sp. in glucose and corn steep liquor containing medium generates approximately 10 to 40 mg zeaxanthin per litre (Sajilata, Singhal & Kamat, 2008). Supplementation with palmitic esters, methionine, pyridoxine, ferrous salts, and reduction in temperature, have can increases the yield to 335 mg per litre.
- Oxygen
Oxygen supply in the culture medium was found to be another important factor in the production of these microbial pigments (Gierhart, 1994). An increase in oxygen supply is generally correlated with an increase in the culture’s productivity. Oxygen is not only required for the growth of Sphingobacterium sp. alone, but also for desaturation, cyclization and oxygenation of carotenoids (Sajilata, Singhal & Kamat, 2008). Zeaxanthin production can be improved by having a high oxygen transfer rate fermentor. The oxygen supply in the fermentor is effectively affected by two factors: agitation speed and aeration rate. The oxygen supply required by the growing culture can be adjusted by having a proper combination of these two factors.
References
Abro, A. H., Rahimi Shahmirzadi, M. R., Jasim, L. M., Badreddine, S., & Al Deesi, Z. (2016). Sphingobacterium multivorum Bacteremia and Acute Meningitis in an Immunocompetent Adult Patient: A Case Report. Iranian Red Crescent medical journal, 18(9), e38750. https://doi.org/10.5812/ircmj.38750
Alcantara, S. & Sanchez, S. (1999). Influence of carbon and nitrogen sources on Flavobacterium growth and zeaxanthin biosynthesis. Journal of Industrial Microbiology & Biotechnology, 23, 697-700.
Garnett, K. M., Gierhart, D. L. & Guerra-Santos, L. H. (1998). Method of making pure 3R-3_R stereoisomer of zeaxanthin for human ingestion. U.S. patent 5,854,015. date of issue Dec 29 1998.
Gierhart, D. L. & Applied Food Biotechnology Inc. (1994). Production of zeaxanthin and zeaxanthin-containing compositions. U.S. patent 5,308,759. date of issue May 3 1994.
Mendes, M. D., Cavallo, R. R., Carvalhães, C. H., & Ferrarini, M. A. (2016). Septic arthritis by Sphingobacterium multivorum in imunocompromised pediatric patient. Artrite séptica por Sphingobacterium multivorum em paciente pediátrico imunossuprimido. Revista paulista de pediatria : orgao oficial da Sociedade de Pediatria de Sao Paulo, 34(3), 379–383. https://doi.org/10.1016/j.rpped.2015.12.001
Ram, S., Mitra, M., Shah, F., Tirkey, S. R., & Mishra, S. (2020). Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. Journal of Functional Foods, 67. https://doi.org/10.1016/j.jff.2020.103867
Sajilata, M. G., Singhal, R.S. & Kamat, M. Y. (2008). The carotenoid pigment
zeaxanthin. Food Science and Food Safety, 7, 29-49.