This article was prepared using DeepSeek-V3 with the following prompt: Write an 800 word blog article on the biochemistry of reactions in triple sugar iron agar. It was then checked/edited by Dr Philip Button.
Triple Sugar Iron (TSI) agar is a differential medium used extensively in microbiology to identify enteric bacteria based on their ability to ferment sugars and produce hydrogen sulfide (H₂S). This versatile medium provides valuable insights into the metabolic capabilities of microorganisms, making it a cornerstone in clinical and environmental microbiology. Understanding the biochemistry of reactions in TSI agar requires a closer look at its composition, the metabolic pathways involved, and the visual indicators that reveal microbial activity. This article explores the biochemical principles behind the reactions observed in TSI agar and their significance in microbial identification.
Composition of Triple Sugar Iron Agar
TSI agar is a complex medium containing three sugars (glucose, lactose, and sucrose), a pH indicator (phenol red), and iron salts. Its composition is designed to test multiple metabolic capabilities of bacteria simultaneously. The key components include:
- Sugars:
- Glucose: 0.1% concentration, a monosaccharide that serves as a primary energy source for most bacteria.
- Lactose: 1% concentration, a disaccharide composed of glucose and galactose.
- Sucrose: 1% concentration, a disaccharide composed of glucose and fructose. The low concentration of glucose relative to lactose and sucrose ensures that glucose is depleted first, allowing for the observation of secondary fermentation reactions.
- Phenol red: A pH indicator that turns yellow under acidic conditions (pH < 6.8) and red under alkaline conditions (pH > 7.4). It helps visualize changes in pH due to sugar fermentation.
- Iron salts: Typically ferrous sulfate or ferric ammonium citrate, which react with hydrogen sulphide (H₂S) to form a black precipitate of ferrous sulphide (FeS).
- Peptone: Provides nitrogen and amino acids for bacterial growth.
- Agar: A solidifying agent that allows for the creation of a slanted surface, providing both aerobic (slant) and anaerobic (butt) conditions.
Biochemical reactions in TSI Agar
The reactions in TSI agar are driven by the metabolic activities of bacteria, including sugar fermentation, gas production, and H₂S generation. These reactions are interpreted based on color changes in the medium and the presence of gas or black precipitates.
1. Sugar fermentation
- Glucose fermentation: Bacteria that ferment glucose produce acidic byproducts (e.g., lactic acid, acetic acid), lowering the pH of the medium. This is indicated by a yellow color in the butt of the tube, where anaerobic conditions favor fermentation. The slant may initially turn yellow but will revert to red as glucose is depleted and oxidative metabolism resumes.
- Lactose and sucrose fermentation: If a bacterium can ferment lactose or sucrose, it will continue to produce acid, maintaining a yellow colour in both the slant and the butt. This indicates that the organism can utilise these disaccharides as energy sources. Organisms that ferment only glucose will produce an alkaline slant (red) and an acidic butt (yellow), creating a “K/A” (alkaline/acidic) reaction. Those that ferment lactose or sucrose will produce an “A/A” (acidic/acidic) reaction.
2. Gas Production
- During fermentation, some bacteria produce gases such as carbon dioxide (CO₂) and hydrogen (H₂). Gas production is indicated by cracks or bubbles in the agar or by the agar being lifted from the bottom of the tube.
3. Hydrogen Sulfide Production
- Certain bacteria, such as Salmonella and Proteus (Figure 1), can reduce sulphur-containing compounds (e.g., thiosulphate) to hydrogen sulphide (H₂S). H₂S reacts with iron salts in the medium to form ferrous sulphide (FeS), a black precipitate. This is observed as a blackening of the butt or along the stab line.
Metabolic pathways involved
The biochemical reactions in TSI agar are governed by specific metabolic pathways:
- Glycolysis and fermentation:
- Glucose is metabolised via glycolysis, producing pyruvate. Under anaerobic conditions, pyruvate is converted into various end products, such as lactic acid, ethanol, or acetic acid, depending on the organism. This process generates ATP and lowers the pH of the medium.
- Lactose and sucrose are first hydrolysed into their monosaccharide components by specific enzymes (lactase and sucrase, respectively) before entering glycolysis.
- Oxidative metabolism:
- In the presence of oxygen (on the slant), bacteria may switch to oxidative metabolism, using the Krebs cycle (Figure 2) and electron transport chain to generate ATP. This process produces CO₂ and water, raising the pH and causing the slant to turn red.
- Sulphur reduction:
- Some bacteria possess enzymes like thiosulphate reductase, which reduce thiosulfate to H₂S. This pathway is often coupled with anaerobic respiration, using sulphur compounds as terminal electron acceptors.
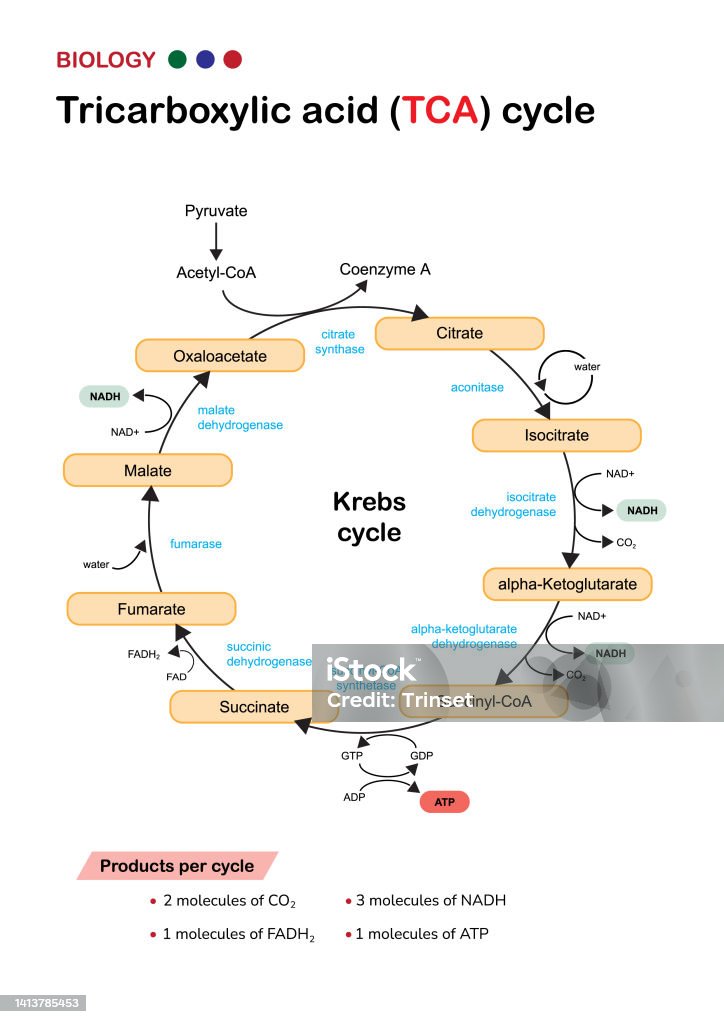
Interpretation of TSI Agar results
The visual changes in TSI agar provide critical information about the metabolic capabilities of the tested organism. An example is shown in Figure 3.
- Yellow Slant and Yellow Butt (A/A):
- Indicates fermentation of lactose and/or sucrose in addition to glucose. Common in Escherichia coli and Klebsiella species.
- Red Slant and Yellow Butt (K/A):
- Indicates glucose fermentation only. Common in Shigella and Proteus species.
- Red Slant and Red Butt (K/K):
- Indicates no fermentation of any sugar. Common in non-fermentative bacteria like Pseudomonas aeruginosa.
- Blackening of the Medium:
- Indicates H₂S production. Common in Salmonella and some Proteus species.
- Gas Production:
- Indicates the production of CO₂ or H₂ during fermentation.

Applications of TSI Agar
TSI agar is widely used in clinical laboratories to identify enteric pathogens, such as Salmonella, Shigella, and E. coli. It is also used in environmental microbiology to study the metabolic diversity of bacteria in various ecosystems. The medium’s ability to test multiple metabolic traits simultaneously makes it a cost-effective and efficient tool for microbial identification.
Conclusion
The biochemistry of reactions in Triple Sugar Iron agar is a fascinating interplay of microbial metabolism and chemical indicators. By understanding the metabolic pathways involved and the visual cues provided by the medium, microbiologists can gain valuable insights into the identity and capabilities of bacterial isolates. TSI agar remains a cornerstone of microbiological diagnostics, demonstrating the enduring relevance of biochemical principles in modern science. Whether in a clinical lab or a research setting, TSI agar continues to be an indispensable tool for unraveling the metabolic secrets of microorganisms.



