By: Jill Koh & Philip Button
This is the first part of a two part series on sodium benzoate. Both were written by an 4th-year Honours student (who completed the program with 1st class Honours, H1) I was supervising at RMIT University in 2010. I have revised and updated were necessary and appropriate.
Food additives are commonly found in processed foods which are used to facilitate or complement a variety of production methods in today’s modern food supply. According to the Food and Drug Administration (FDA), a food additive is defined as “any substance the intended use of which results or may reasonably be expected to result- directly or indirectly, in its becoming a component of or otherwise affecting the characteristics of any food”. Food additives carry out three basic functions (Figure 1): I) to maintain food safety, spoilage and quality by preventing microbial growth or chemical changes, II) improve or maintain nutritional value and III) improve taste, texture and appearance (Emerton & Choi, 2008; Food and Drug Administration, 2007).
Preservatives are considered one of the most important classes of food additives as they play an important role in safety of the food supply. Preservatives are added to foods because elimination of potential microbial contamination through physical methods is not viable (de Mendonça, Vaz, & de Mendonça, 2001). Employment of chemical preservatives such as salt (Figure 2), spices and sulphite have been age-old practices to preserve perishable foods before the advent of refrigeration and processing techniques (Emerton & Choi, 2008) which are able to extend the shelf-life of (primarily) perishable food. Food preservatives extend the shelf-life of foods with their antimicrobial activity to prevent food poisoning (Food and Drug Administration, 2007; Mc Williams, 2008). Hence, they offer consumers the benefit of keeping food safe over the shelf-life of the product to meet the demands of modern lifestyles.
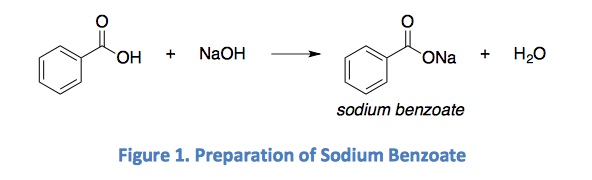
Sodium benzoate, which is food additive E211, is a salt of benzoic acid commonly used for preservation purposes in a wide range of foods such as margarine, salad dressings, sauces and especially carbonated beverages/juices (Figure 3) although it is also naturally found in some fruits such as cranberries and prunes (Food and Drug Administration, 2007; International Programme on Chemical Safety; Jay, 2000). Listed as a GRAS (Generally Recognised as Safe) chemical, sodium benzoate is produced by neutralisation of benzoic acid with sodium hydroxide (Figure 4) and effectively inhibits yeasts and mould growth (International Programme on Chemical Safety; Jay, 2000; Krebs, Wiggins, & Stubbs, 1983). Sodium benzoate is usually preferred by food manufacturers due to its high solubility in aqueous solutions (200 times more soluble) as opposed to benzoic acid (Gardner & Lawrence, 1993; International Programme on Chemical Safety). However, due to the distinctive off-flavour imparted to foods at high concentrations, sodium benzoate has a maximum tolerance level of 0.1% in the US (de Mendonça, et al., 2001; International Programme on Chemical Safety; Jay, 2000), although the World Health Orgnization has stipulated a maximum limit of 0.2% (Wibbertmann et al., 2000).
The antimicrobial activity of sodium benzoate is related to environmental pH, displaying greatest activity at low pH values. Extensive literature work has revealed effectiveness of sodium benzoate against a range of food spoilage yeasts such as S.cerevisiae, Z.rouxii, and Y.lipolytica (Praphailong & Fleet, 1997). Guynot et al. (2005) has also reported efficacy of sodium benzoate in preventing Aspergillus spp. and Penicillium corylophilum spoilage in bakery products at low aw levels. The mechanism of antimicrobial action of sodium benzoate was reported by Jay (2000), Hazan et al. (2004) and Krebs et al. (1983) – under acidic conditions, the undissociated form of sodium benzoate enters the cell and acts as a proton ionophore by facilitating proton leakage into cells. Intracellular acidification of cells inhibit cellular uptake of substrate molecules (such as amino acid transport), resulting in oxidative stress and autophagy, hence inhibiting cell growth.
This offers an introduction to chemical food preservatives and in particular, the uses and mechanism of action of this common additive. In the second blog post in this series, we shall explore the safety of sodium benzoate.
References:
de Mendonça, A. J. G., Vaz, M. I. P. M., & de Mendonça, D. I. M. D. (2001). Activity coefficients in the evaluation of food preservatives. [doi: DOI: 10.1016/S1466-8564(01)00037-6]. Innovative Food Science & Emerging Technologies, 2(3), 175-179.
Emerton, V., & Choi, E. (Eds.). (2008). Essential Guide to Food Additives (3rd ed.). Leatherhead, UK: Leatherhead Publishing.
Food and Drug Administration. (2007, 18 May 2009). Data on Benzene in Soft Drinks and Other Beverages. Retrieved 4 May 2010, from http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Benzene/ucm055815.htm#table2
Gardner, L. K., & Lawrence, G. D. (1993). Benzene production from decarboxylation of benzoic acid in the presence of ascorbic acid and a transition-metal catalyst. [doi: 10.1021/jf00029a001]. Journal of Agricultural and Food Chemistry, 41(5), 693-695.
Guynot, M. E., Ramos, A. J., Sanchis, V., & Marin, S. (2005). Study of benzoate, propionate, and sorbate salts as mould spoilage inhibitors on intermediate moisture bakery products of low pH (4.5–5.5). International Journal of Food Microbiology, 101(2), 161-168.
Hazan, R., Levine, A., & Abeliovich, H. (2004). Benzoic acid, a weak organic acid food preservative, exerts specific effects on intracellular membrane trafficking pathways in Saccharomyces cerevisiae. Appl. Environ. Microbiol., 70(8), 4449-4457.
International Programme on Chemical Safety. BENZOIC ACID AND SODIUM BENZOATE. Retrieved 4 May 2010, from http://www.inchem.org/documents/cicads/cicads/cicad26.htm#SectionNumber:4.1
Jay, J., M. (2000). Modern Food Microbiology (6th ed.): Springer-Verlag.
Krebs, H., A., Wiggins, D., & Stubbs, M. (1983). Studies on the mechanism of the antifungal action of benzoate. The Biochemical Journal, 214, 657-663.
Mc Williams, M. (2008). Foods: Experimental Perspectives (6th ed.). New Jersey: Pearson Education.
Praphailong, W., & Fleet, G. H. (1997). The effect of pH, sodium chloride, sucrose, sorbate and benzoate on the growth of food spoilage yeasts. [doi: DOI: 10.1006/fmic.1997.0106]. Food Microbiology, 14(5), 459-468.
Wibbertmann, A., Kielholm, J., Koennecker, G., Mangelsdorf, I. & Melber, C. (2000). Benzoic acid and sodium benzoate. https://www.who.int/ipcs/publications/cicad/cicad26_rev_1.pdf






North American food preservative segment remained stable during this quarter, backed by firm offtakes from the downstream end users. Food preservative sector of USA usually remained stable, due to strong market movement and the extensive use of preservatives in the country. However, despite of this stability, price of Sodium Benzoate Pricing witnessed a marginal hike during this timeframe, due to rise in feedstock Benzoic Acid prices. The shortage of feedstock Benzoic Acid was supported by prolonged lower inventories level, while manufacturers filled their back orders.
https://www.chemanalyst.com/Pricing-data/sodium-benzoate-1185
Many thanks for your insights in this area chemanalyst.
This is the first part of a two part series on food sources
Food additives are commonly found in processed foods which are used to facilitate or complement a variety of production methods in today’s modern foo
This first part of the series on sodium benzoate provides a thorough overview of its role as a food preservative. Sodium benzoate, found in items like margarine and carbonated beverages, is praised for its antimicrobial properties, which help extend the shelf life of foods. The article also explains its mechanism of action and effectiveness at low pH levels. For more on sodium benzoate’s price trends, check here. Eagerly awaiting the next part, which will focus on the safety of sodium benzoate.