This article is an extract from a literature review written by Louise Sidharta, a 3rd-year undergraduate student I was supervising at RMIT University in 2009.
Introductory comments by Dr Philip Button. For probiotic bacteria to exert their positive effects, they need to survive many hostile (micro)environments before they reach the location in the gastrointestinal tract where they are needed. Food processing conditions, temperate fluctuations during the post-manufacture supply chain, intrinsic conditions in the food and the gastrointestinal tract all provide challenges. To deliver the maximum amount of probiotics right their site of action can pose a great challenge to food microbiologists – these are some of the strategies they can employ to ensure the highest percentage of survival. Let’s now hear from Louise…
Strain selection
As mentioned previously, the ability to survive through the acid in the human stomach and bile in the intestine is one of the most important characteristics of probiotic bacteria. A number of studies on the survival of L. acidophilus (Figure 1) and Bifidobacterium spp. in the presence of acid and bile salts have been carried out by several investigators (Conway, Gorbach, & Goldin, 1987; Gilliland, Staley, & Bush, 1984; Holcomb, Frank, & McGregor, 1991; Ibrahim & Bezkorovainy, 1993). Clark & Martin (1994) reported that B. longum tolerated bile concentrations of as high as 4.0%, whereas Ibrahim & Bezkorovainy (1993) found B. longum to be the least resistant to bile. Furthermore, three out of six CSIRO strains of lactobacilli showed the best survivability under acidic conditions and two strains showed the best tolerance to bile (Lankaputhra & Shah, 1995). Therefore, careful selection of strains on the basis of acid and bile tolerance would help improve the survivabilityensure the required minimum amount of probiotic bacteria in a given product.

Two-step fermentation
The composition of the yoghurt cultures participating in the fermentation has been suspected postulated to affect the survival of L. acidophilus and Bifidobacterium spp., causing their severe viability losses during storage (Shah, 2000). Inhibitory substances such as acid and hydrogen peroxide produced by yoghurt cultures are the major cause of this problem, as explained by Hull, Roberts, & Mayes (1984). Nevertheless, these cultures are prerequisite in yoghurt manufacture as they are responsible for the typical yoghurt flavour. Lankaputhra & Shah (1997) suggested two-stage fermentation as means of improving the viability of probiotic bacteria. Results from this study showed that fermentation with probiotic bacteria followed by fermentation with yoghurt cultures brings a significant increase in the initial and final counts of probiotic bacteria. The initial counts have been found to increase by 4 to 5 times and the final counts of L. acidophilus 2409 and B. longum 1941 following 6 weeks of storage were 6.85 and 7.93 log cfu/g and 7.60 and 8.84 cfu/g for yoghurt prepared by single and two-stage fermentation, respectively.
Oxygen impermeable packaging
L. acidophilus is microaerophilic and Bifidobacterium spp. are strictly anaerobic. Oxygen affects the probiotic cultures in two ways; by direct or indirect toxicity to cells (Dave & Shah, 1997). Direct toxicity is presumably due to the intracellular production of hydrogen peroxide, while indirect toxicity is caused by the production of hydrogen peroxide by other cultures in the environment such as L. delbrueckii (Villegas & Gilliland, 1998). One way to prevent the detrimental effects of oxygen on the probiotic cultures is through the use of oxygen-impermeable packaging. Dave & Shah (1997) reported a higher increase in numbers and survival of L. acidophilus during storage in yoghurts made in plastic containers rather than glass. Bifidobacteria showed higher survival in glass bottles than in plastic cups. To be exact, the initial counts of the bifidobacteria population were 1.6 fold higher in yoghurt prepared in glass bottles than plastic cups, although the acid contents were similar in both samples. Klaver et al. (1993) observed better survival and viability of bifidobacteria in deaerated milk.
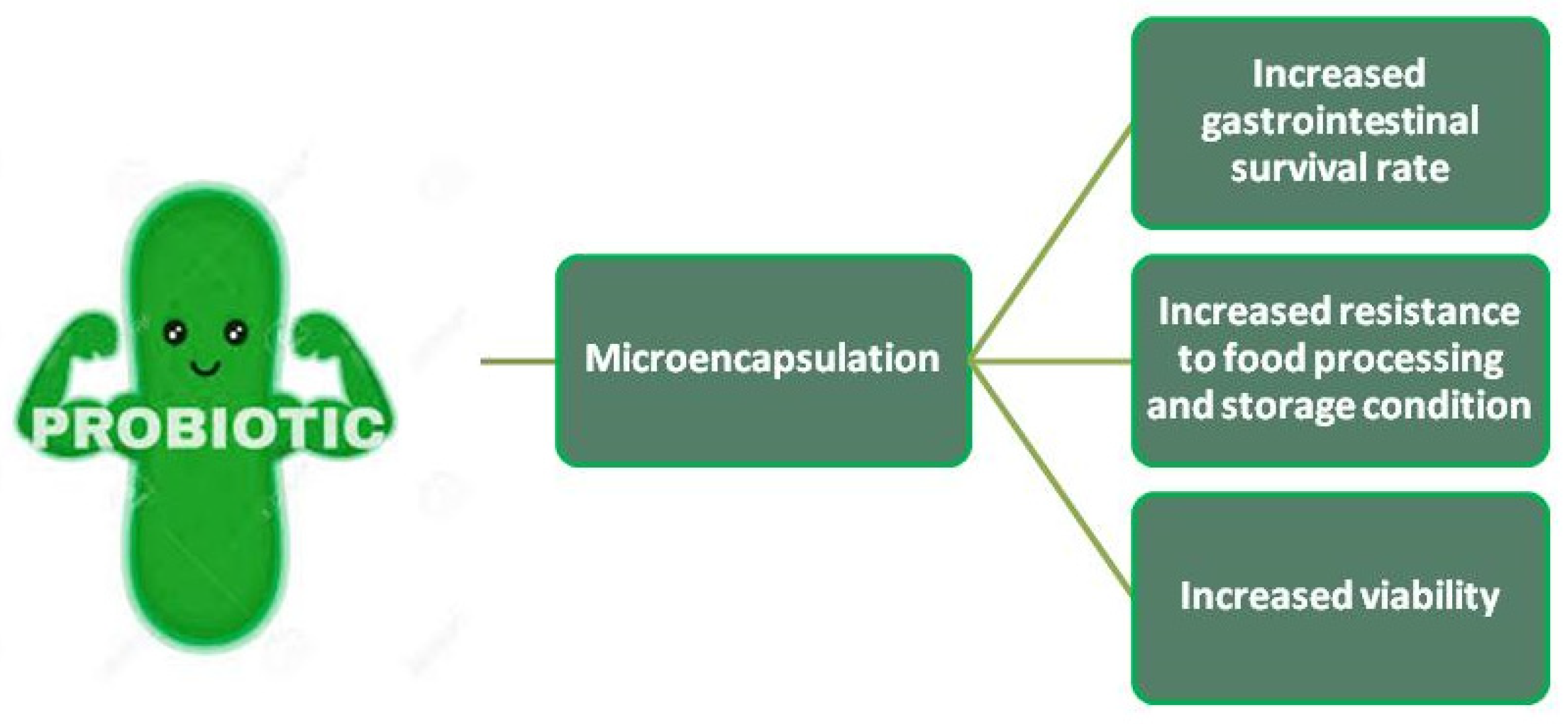
Microencapsulation
Microencapsulation technology has been under special consideration and investigation as an effective approach to enhance the survival rate of probiotic bacteria in acidic products (Figure 2) such as dairy fermented products (Kailasapathy, 2002; Krasaekoopt, Bhandari, & Deeth, 2003). Shahidi & Han (1993) define microencapsulation as “a technology of packaging solids, liquid or gaseous materials in miniature, sealed capsules that can release their contents at controlled rates under the influence of specific conditions”. Another definition from a microbiological point of view defines microencapsulation as “the process of entrapment/enclosure of microbial cells by means of coating them with proper hydrocolloid(s) in order to segregate the cells from the surrounding environment; in a way that results in appropriate cell release in the intestinal medium” (Sultana et al., 2000; Krasaekoopt et al., 2003; Picot & Lacroix, 2003). Microencapsulation has been shown to protect the probiotic bacterial cells against the influences of adverse environmental factors such as high acidity and low pH (Sun & Griffiths, 2000), bile salts (Lee & Heo, 2000), cold shocks caused by deep freezing and freeze drying (Shah & Ravula, 2000), molecular oxygen in case of strictly anaerobic microorganisms like Bifidobacterium spp. (Sunohara et al., 1995), and heat shocks caused by spray drying (Steenson et al., 1987). According to Anal & Singh (2007), the most commonly used encapsulant materials in microencapsulation are food-grade polymers such as alginate, chitosan, κ-carrageenan and gelatin.
References
Anal, A. K. & Singh, H. (2007). Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends in Food Science and Technology, 18, 240-251
Conway, P. L., Gorbach, S. L., & Goldin, B. R. (1987). Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. Journal of Dairy Science, 70, 1-4
Clark, P. A. & Martin, J. H. (1994). Selection of bifidobacteria for use as dietary adjuncts in cultured dairy foods: Tolerance to simulated bile concentrations of human small intestines. Cultural Dairy Products Journal, 29, 18-21
Dave, R. I. & Shah, N. P. (1997). Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. International Dairy Journal, 7, 31-41
Gilliland, S. E., Staley, T. E., & Bush, L. J. (1984). Importance of bile tolerance of Lactobacillus bifidus var. pennsylvanicus produced by a cell wall precursor. Biochemistry, Biophysics, 37, 361-363
Holcomb, J. E., Frank, J. F., & McGregor, J. U. (1991). Viability of L. acidophilus and Bifidobacterium bifidum in soft serve frozen yogurt. Cultural Dairy Products Journal, 26, 4-5
Hull, R. R., Roberts, A. V., & Mayes, J. J. (1984). Survival of Lactobacillus acidophilus in yogurt. Australian Journal of Dairy Technology, 39, 164-166
Ibrahim, S. A. & Bezkorovainy, A. (1993). Inhibition of Escherichia coli by bifidobacteria. Journal of Food Protection, 56, 713-715
Kailasapathy, K. (2002). Microencapsulation of probiotic bacteria: technology and potential applications. Current Issues in Intestinal Microbiology, 3(2), 39-48
Klaver, F. A. M., Kingma, F., & Weerkamp, A. H. (1993). Growth and survival of bifidobacteria in milk. Netherlands Milk and Dairy Journal, 47, 151-164
Lankaputhra, W. E. V. & Shah, N. P. (1997). Improving viability of Lactobacillus acidophilus and bifidobacteria in yogurt using two step fermentation and neutralised mix. Food Australia, 49, 363-366
Lankaputhra, W. E. V. & Shah, N. P. (1995). Survival of Lactobacillus acidophilus and Bifidobacterium species in the presence of acid and bile salts. Cultured Dairy Products Journal, 30(3), 113-118
Lee, K. I., & Heo, T. R. (2000). Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juices and bile salt solution. Applied and Environmental Microbiology, 66, 869-973
Picot, A., & Lacroix, C. (2003). Effect of micronization on viability and thermotolerance of probiotic freeze-dried cultures. International Dairy Journal, 13, 455-462
Shah, N. P. (2000). Probiotic bacteria: Selective enumeration and survival in dairy foods. Journal of Dairy Science, 83, 894-907
Shah, N. P., & Ravula, R. R. (2000). Microencapsulation of probiotic bacteria and their survival in frozen fermented dairy desserts. Australian Journal of Dairy Technology, 55(3), 139-144
Shahidi F, & Han X.POPAROONI. (1993). Encapsulation of food ingredients. Crit Rev Food Sci Nutr. 33(6):501-547. doi:10.1080/10408399309527645
Steenson, L. R., Klaenhammer, T. R., & Swaisgood, H. E. (1987). Calcium alginate-immobilized cultures of lactic streptococci are protected from attack by lytic bacteriophage. Journal of Dairy Science, 70, 1121-1127
Sultana, K., Godward, G., Reynolds, N., Arumugaswamy, R., Peiris, P., & Kailasapathy, K. (2000). Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. International Journal of Food Microbiology, 62, 47-55
Sun, W. R., & Griffiths, M. W. (2000). Survival of bifidobacteria in yoghurt and simulated gastric juice following immobilization in gellan-xanthan beads. International Journal of Food Microbiology, 61, 17-26
Sunohara, H., Ohno, T., Shibata, N., & Seki, K. (1995). Process for producing capsule and capsule obtained thereby. U.S Patent, 5, 478-570
Villegas, E. & Gilliland, S. E. (1998). Hydrogen peroxide production by Lactobacillus delbrueckii subsp. lactis at 5°C. Journal of Food Science, 63, 1070-1075

