In 1998 and 1999, at what is now the Mount Helen campus of Federation University in Ballarat, Victoria, I completed my Honours degree in food science, submitting my thesis on 16 July 1999 and graduating in May the following year. My thesis was in the area of bacterial physiology, specifically on the adaptive acid tolerance response in two key food borne disease bacteria – the Gram negative Escherichia coli and the Gram positive Staphylococcus aureus. Many physiological mechanisms may contribute to this acid tolerance response, which has much significance in food manufacturing and indeed the virulence of these bacteria, which ultimately, makes this physiological phenomenon of public health significance too. An increased acid tolerant state may offer enhanced survival in low pH foods and aid subsequent gastric passage, enabling proliferation further along the gastrointestinal tract. Thus, the adaptive acid tolerance response is considered a virulence factor as it contributes to pathogenesis. In this blog article, I want to cover just two of the (genetic) bases for an adaptation to extremes and normally lethal pH values. Please note though that my thesis was submitted in July of 1999, and therefore this piece of writing covers the knowledge in this area up to that date only.
4.7.2 Genes
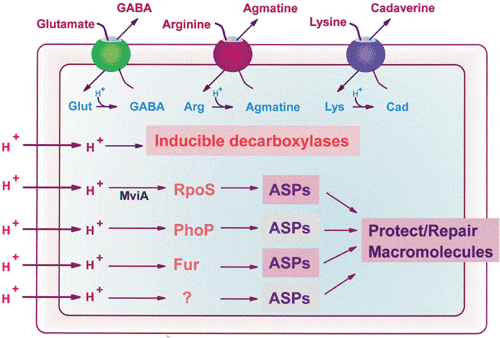
A number of genes have been identified which directly or indirectly assist bacteria in their attempt to survive low pH conditions. With S. typhimurium, at least 17 genes have been identified (Foster, 1995). These are reviewed by Foster (1995). Of these, two genes, rpoS and fur will be discussed further.
Some genes, present in a variety of bacterial species, are widely accepted as regulators of inducible acid tolerance, such as rpoS and probably fur. However, some specific genes may play an essential role in the inducible acid tolerance of certain bacteria, for example, actA in Rhizobium meliloti (Tiwari et. al., 1996).
4.7.2.1 rpoS gene
The rpoS gene is a member of a group of genes that enable survival under a range of extreme conditions. A related gene, also widely researched (Huang et. al., 1998; Emetz and Klug, 1998), is rpoH, required for the heat shock response of E. coli. Cheville et al. (1995) have demonstrated the vital nature of rpoS in a variety of acidic conditions, including fermented meat, and in addition, found that the stationary phase RpoS protein gave tolerance to heat and salt. Salmonella have also been shown (through gene cloning experiments) to require rpoS to tolerate acid (Lee et. al., 1995) while similar observations have been made with Bacillus subtilis (Hecker and Volker, 1998). More importantly, the alternative sigma factor encoded by rpoS and the subsequent acid shock proteins, such as RpoS, induced a sustained ATR rather than just a transient induced tolerance to acid (Lee et. al., 1995). They have shown that induction of the transient ATR can occur without RpoS but for the cells to survive beyond 20min in low pH, RpoS is required. The central role played by the rpoS gene has been clearly demonstrated in a number of species by the construction of mutants lacking this gene. These species include S. flexneri (Waterman and Small, 1996) and S. Typhimurium (Wilmes-Risenberg et. al., 1997). As well as enabling survival in extreme heat, salt and acid, alternate sigma factors (in this situation, alternate sigma factor 54) can also function in times of nitrogen deprivation of the cell via the gene rpoN, as described by Lewin (1994). While these groups of genes are responsible for enabling survival in a variety of stress responses, rpoS itself can provide general stress protection against diverse environmental conditions. For example, in Pseudomonas aeruginosa, rpoS increases resistance up to three-fold against heat, low pH, high osmolarity, hydrogen peroxide and ethanol (Jorgensen et. al., 1999).
4.7.2.2 fur gene
Responsible for iron uptake, the fur (ferric uptake regulator) gene has been shown by Lee et. al. (1995) to be necessary for a transient ATR in S. Typhimurium while Foster and Hall (1992) describe the contribution of the Fur protein to expression of fur as “extensive”. Bagg and Neilands (1987) describe the action of Fur and state that the most obvious means of action is that transcription of fur is repressed by Fur binding to Fe, which reversibly attaches to the operator sequence. Perhaps unexpectedly, the ATR function of Fur is not induced by iron with both systems (iron acquisition and ATR) acting independently of each other (Hall and Foster, 1996). S. Typhimurium fur mutans demonstrate the importance of this gene as shown by the work of Foster (1993) who has shown that a fur mutant was unable to induce pre- or post-shock acid adaptation and also by Wilmes-Riesenberg et. al. (1996) who found that this mutant had reduced virulence as the LD50 (in mice) was higher, up to a 3-log increase. Although not a direct mutation in fur, Foster and Bearson (1994) have shown that S. Typhimurium mutants with mutations in atrD and atrF (these mutations affect iron metabolism and regulation) are acid-sensitive. They state that this supports the idea of the involvement of Fur-regulated genes in inducible acid tolerance.