Introduction
Food preservation is generally about incorporation of multiple hurdles (Figure 1) which will either slow microbial growth, stop microbial growth or eliminate microorganisms. Multiple hurdles are required because of the diversity of microbial contaminants likely to be present in a given food product, For example, the parameters dictating the growth of yeasts and mesophilic bacteria are quite different. Different bacteria might be very different in this regard, as could be different yeasts and different moulds from one another. Therefore, apart from achieving what is known as ‘commercial sterility’ in your product (and even this has exceptions, particularly with regard to spore forming bacteria), no other preservation approach can be used for wide spectrum application against all the ‘troublesome’ microorganism that might be encountered. Hurdles can generally be classified as intrinsic (factors present within the food) or extrinsic (factors present outside of the food) and the three discussed below are generally the most important for preservation of most food products. Therefore, these three factors could be reasonably expected to contribute to preservation of many products.
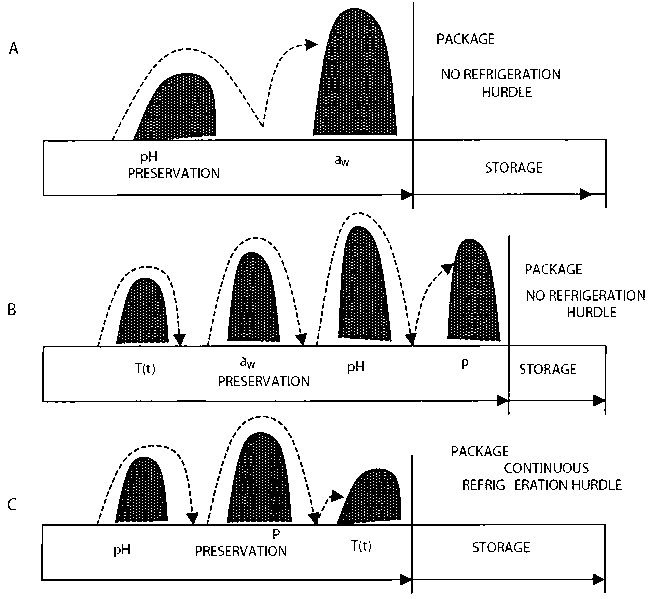
Figure 1: Hurdle concept in food preservation
Temperature – extrinsic factor
Many people will recognise that temperature control is key to extending the keeping quality of food. This is because, with some exceptions, the micro-organisms that will be most problematic from a safety and quality perspective will grow well within a certain range. While this range of temperature for optimal growth relatively narrow, at roughly 25 °C to 35 °C, the temperature danger zone is an important concept in food microbiology, where between 5 °C and 60 °C, any contaminating microorganisms can be expected to grow, given long enough. Important exceptions to this would be Listeria monocytogenes, which is a a psychotropic pathogen and various Pseudomonas species, such as P. fluorescens and P. fragi, which are psychotropic spoilage bacteria. Psychrotrophy is a key concept in shelf-life (and is different to microorgniams being psychrophiles – Figure 2), since low temperature storage doesn’t stop spoilage or unsafe food from developing, but merely delays it and in fact selects for the growth of these bacteria. Therefore, while refrigeration is an excellent means to extend shelf-life, one must be mindful of the possible presence of psychotropic microorganisms and their dominance in refrigerated foods over time.
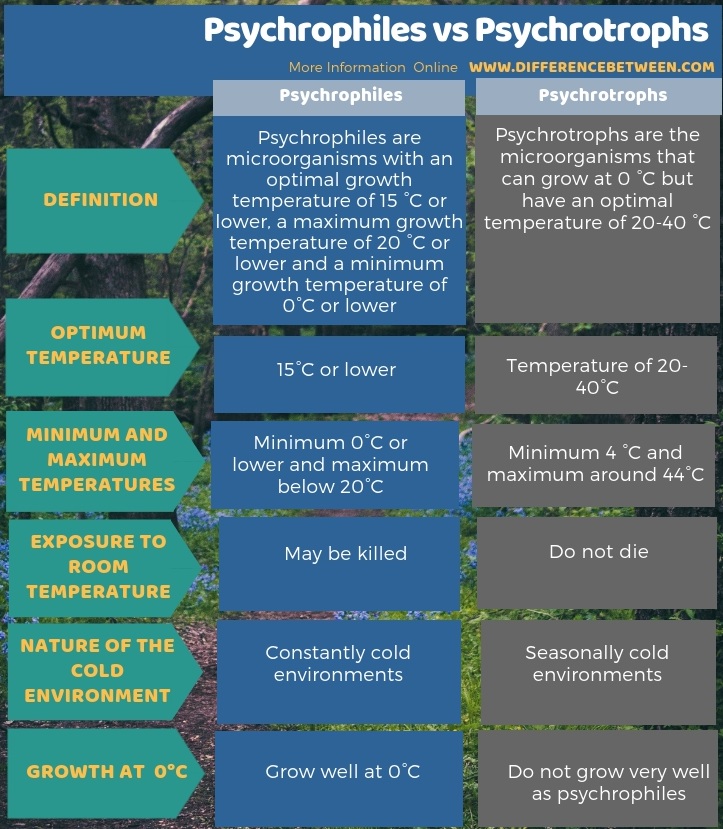
Figure 2: Difference being psychotropic and psychrophillic growth.
pH – intrinsic factor
The degree of acidity can be considered on the pH scale, with different microorgiansms growing at different pH values and over a different range of pH values (Figure 3). An important consideration of intrinsic product hurdles to microbial growth is that not all hurdles that appear the same, are the same. For example, there are significant differences in the environment when different acids are used as acidulent. Therefore, a pH of 3.0 or 4.0 will have variation in severity with regard to its antimicrobial capabilities when an inorganic or organic acid is used. Of course, only organic acids are permitted in foods, but even with organic acids, there are variations between common types. Citric acid permits growth of microorganisms down to a pH of 2.1, but if acetic acid is the acidulent, then the minimum growth pH is 2.9 – a most significant difference of 0.8 of a pH unit, especially when one considers the pH scale is logarithmic. Interesting research findings of Juven (1976) illustrate this: an acid-tolerant canned fruit spoilage species, Lactobacillus brevis, was tested in media with different acids to effect the same pH. When citric, hydrochloric, phosphoric and tartaric acids were used, this species could grow to a pH of 3.0. However, when lactic acid was used to lower the pH, no growth occurred below pH 3.7 while with acetic acid being used to lower the pH, the minimum growth pH was 4.0.

Figure 3: pH range for microbial growth.
Water activity – intrinsic factor
Water activity, designated aw, is another key growth determinant, but one which, for the majority of foods, is irrelevant because most foods have a level of water that allows microbial growth to occur. To briefly outline, water is present in two forms – bound and unavailable or unbound water that is freely available for microbial growth. The measure of the amount of unbound and freely available water is water activity. This is different from moisture, which is the total amount of water in a product, in this context. Most foods have a high water activity, and this is what most microorganisms require. However, similar to the situation that can occur with pH, once the water activity drops to a relatively low level, and growth of the majority of microorganisms is inhibited (Figure 4), growth of those that are able to grow in those low water activity levels is selected for. In many cases, the organisms that can grow at low pH can also grow at low water activity, so a juice concentrate is therefore particularly suitable for them, especially when competition is removed in the form of many bacteria, which are fast-growing in comparison and would otherwise outcompete mould. Addition of salts (for example sodium chloride) or sugars (for example sucrose) is the standard means to alter water activity in manufactured foods. Thus, the action of concentration of a substrate by removing available water, leads to a decrease in water activity through concentration of the salts or sugars present.

Figure 4: Minimum water activity for microbial growth.
Reference
Juven, B. J. (1976). Bacterial spoilage of citrus products at pH lower than 3.5. Journal of Milk and Food Technology, 39(12), 819-822.

